Valence Electrons and Their Interactions Determine the
It is fascinating to know how many valence. The valence electrons are the electrons that determine the most typical bonding patterns for an element.

Electronic Properties Of Condensed Matter Are Often Determined By An Intricate Competition Between Kinetic Quantum Wave Function Physical Properties Of Matter
In the central part there is a nucleus and electrons move nearby.
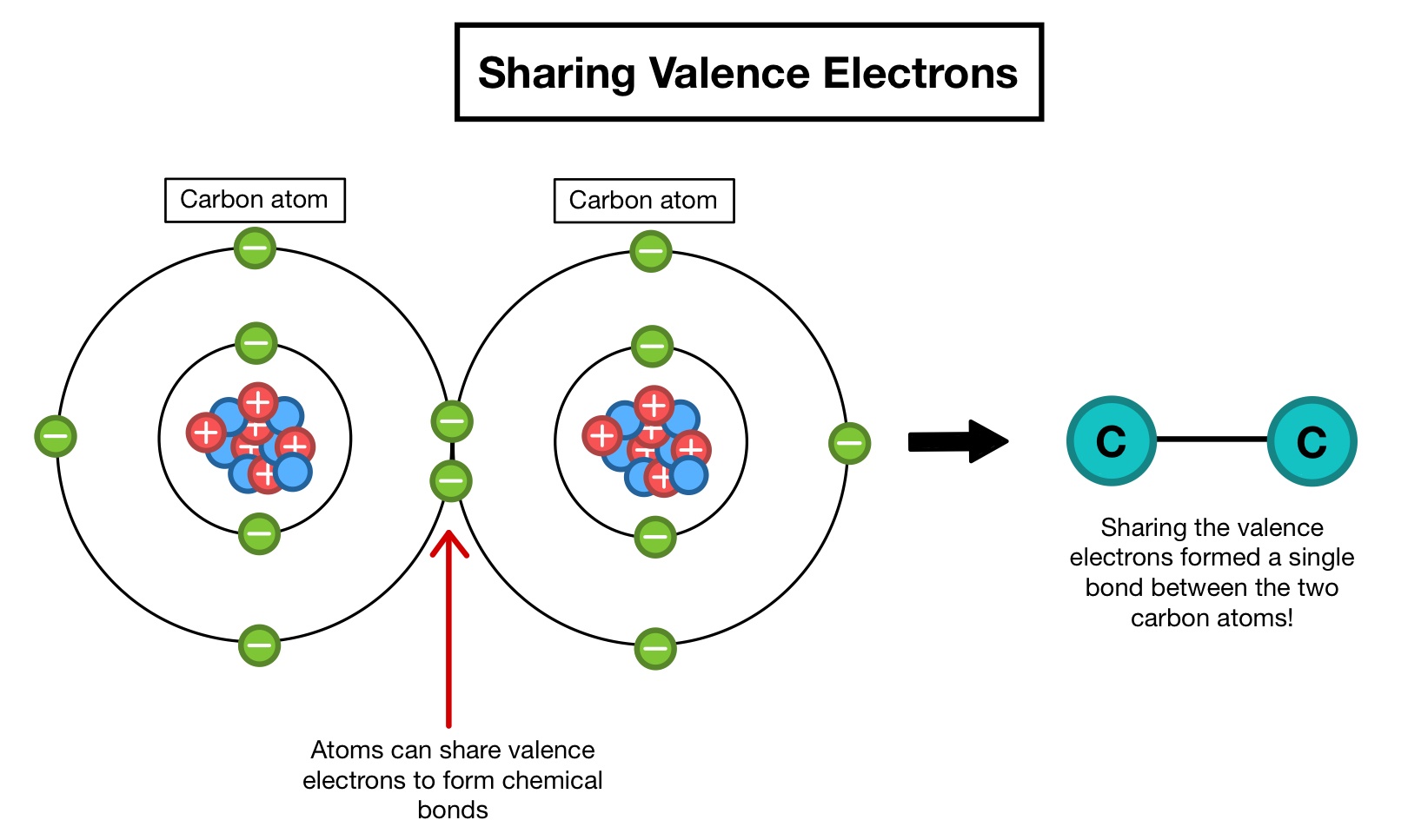
. Valence electrons are the electrons in the outermost shell of the atom. To predict molecular shape we usually use a theory called the valence shell electron pair VSEPR repulsion theory. Electrons in the outermost shell are called valence electrons because it is their interactions that determine the chemical properties of an element.
A stable molecule has the same number of electrons in its valence shell as its parent atom. For example valence electrons are. What is NOT true about the valence electron shell.
Electrons in the outermost shell are called valence electrons because it is their interactions that determine the chemical properties of an element. In chemistry valence electrons are the electrons that are located in the outermost electron shell of an element. An atom has some electrons placed outside the energy level and we call them valence electrons.
Chemical Elements possessing all eight electrons two for helium in their energy level are said to be choose all that may apply noble gasses inert. Valence electrons are the electrons located at the outermost shell of an atom. O When unfilled it can interact with other atoms to form chemical bonds.
In this way a given elements reactivity is highly dependent upon its electronic configuration. Lets use methane molecule to further explain this. The columns that were set up to group elements by similar chemical properties turn out to be the exact same columns defined by the number of valence electrons.
3 2 pts Question 43 What is the purpose of an antibiotic-resistance gene in a plasmid. Because their electron clouds are negatively charged pairs of electrons in a valence shell repel one another. The electrons involved in the formation of chemical bonds are called valence electrons.
Electrons in the outermost shell are called valence electrons because it is their interactions that determine the chemical properties of an element. The number of valence shell electron pairs surrounding the core atom determines the structure of a molecule. Additionally there are some interactions between atoms and these valence electrons.
The columns that were set up to group elements by similar chemical properties turn out to be the exact same columns defined by the number of valence electrons. So this compound has 20 valence electrons. The columns that were set up to group elements by similar chemical properties turn out to be the exact same columns defined by the number of valence electrons.
One atom can interact with other atoms. Valence electrons are what determine how atoms bond to each other. Moreover they are quite far from the nucleus.
What are valence electrons and how are they determined. How can you determine the number of valence electrons from an electron configuration. Because when two atoms interact the electrons in the outermost shells are the first ones to come into contact with each other and are the ones that determine how an atom will react in a chemical reaction.
The easiest way to determine valence electrons is by checking out the elements place in the periodic table. It always holds a maximum of 8 electrons. What are valence electrons.
Do valence electrons interact with other atoms. The next one is NBR three which contained nitrogen and bro mean The group number of nitrogen is 15 and that of Roman is 17. When moving top to bottom the of valence electrons decreases because the electronegativity is low.
How do you determine valence electrons. This theory is based on the idea that valence electrons in a molecule tend to repel each other to create more space around them. Sodium has 1 valence electron from the 3s orbital.
There are two atoms of romaine to remain contributes Total 14 electrons from the Valence Shell. The molecules of water in our bodies are held together by covalent bonds because of the valence electrons which are shared between hydrogen and oxygen atoms. Valence electrons and their interactions determine the _____ properties of an element.
As a result chemical bonds are formed. An atom like our solar system has the following structure. A link between 2 atoms resulting from the mutual attraction of their nuclei for valence electrons.
It will determine the chemical behavior of an atom. Unit 4- Atomic Interaction Chapters Combined. Why are these electrons special.
So the other nucleus atom attracts them more compared to their nucleus. When moving left to right on the periodic table the of valence electrons increases because the electronegativity is high. Another way to find or determine valence electrons is through the atomic number.
These electrons are found in the s and p orbitals of the highest energy level for the element. Molecular shape of methane. Knowing how to find the number of valence electrons in a particular atom is an important skill for chemists because this information determines the kinds of chemical bonds that it can form and therefore the elements reactivity.
Valence electrons are the electrons located at the outermost shell of an atom. So now the total number of valence electrons in SBR to our randi. Because when two atoms interact the electrons in the outermost shells are the first ones to come into contact with each other and are the ones that determine how an atom will react in a chemical reaction.
The atomic number of an element number of electrons number of protons. The valence electrons of molecules give those molecules many of their properties. The presence of valence electrons can determine the elements chemical properties such as its valencewhether it may bond with other elements and if so how readily and with how many.
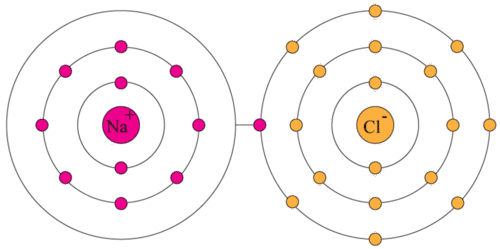
Valence Electrons Ck 12 Foundation

Next Generation Science Standards Ngss Ls2 Ecosystems Interactions Energ Next Generation Science Standards Printable Activities For Kids Responsive Classroom
Comments
Post a Comment